Historically, there is great interest in knowing “during the heat of the day, how high can the pressure go, if my pipe is blocked in?”
To get an idea of the pressure rise on ambient heating of a packed liquid you divide the absolute values of the cubic thermal expansion of the fluid:
α = (1/V)[(∂V/∂T) at constant pressure]
by its isothermal compressibility:
κ = -(1/V)[(∂V/∂P) at constant temperature]
for your ambient temperature range.
α and κ can be looked up, estimated, or measured. α from density changes, and κ from the speed of sound in the liquid.
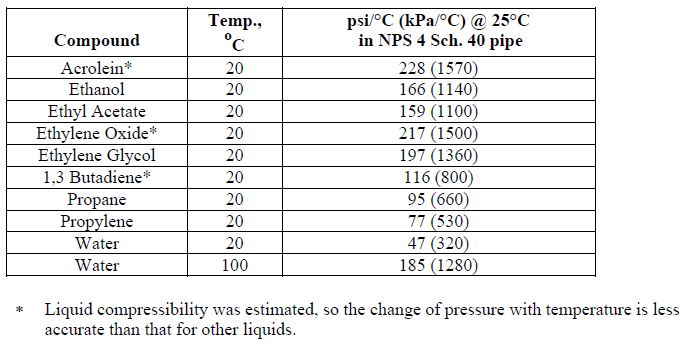
Here is a table of α / κ values from a company reference manual: