QUESTION
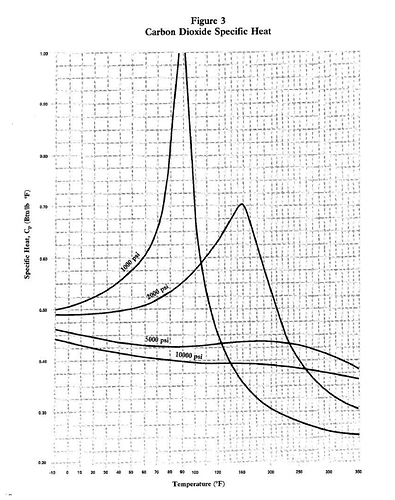
Can anybody explain why liquid CO2 has such a temperature dependant heat capacity, especially at lower pressure (~1,000 psi - see attached graph)?
From the PVT diagram I don’t see any phase changes at the peak heat capacities.
I am planning a job where we will be injection liquid CO2 into an oil well, but because of wellhead limitations, the CO2 needs to be heated before entering the well.
Please see the graph below:
REPLIES
gruntguru
Saturation temperature at 1000 psi is about 80 deg F so there is a phase change from supercritical gas to liquid as you cool through the critical temperature. (Approximate because I am reading off a metric chart and converting)
SOURCE
https://www.eng-tips.com/viewthread.cfm?qid=293579
Above is a snippet.