I co-authored an SDS for several mixtures containing between 50% and 98% ethylene glycol. These are coolant/antifreeze products.
I included target organ toxicity statements and pictograms for kidney and liver damage because…well that’s what ethylene glycol does. I found a multitude of comparable product SDSs to make sure my designations were correct and I was not overstating the hazards nor omitting hazards.
I just received notice from the DLA that the SDSs are non-compliant with GHS and OSHA regulations describing those target organ toxicity statements as “extra”.
I cannot fathom how this is possible. The toxic effects of ethylene glycol are known…well known.
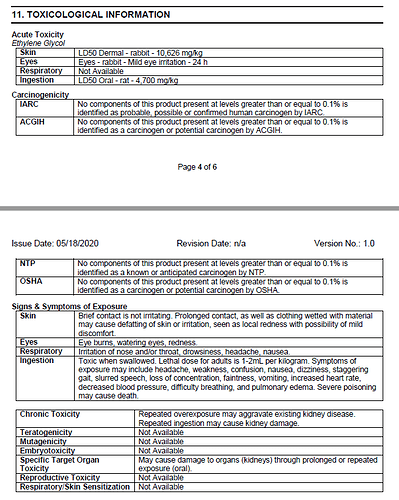
- It is known that prolonged exposure damages the kidneys and the liver.
- Every other manufacturer’s comparable product has the exact same wording and pictogram I am being told is unnecessary and non-compliant.
Please, can anyone provide any insight into this? They are claiming only the exclamation point “warning” for irritant and acute toxicity is required and I cannot find any comparable examples nor figure out how the chronic toxicity of ethylene glycol can be ignored.
Sorry, Defense Logistics Agency (Military agency for purchasing materials)
As a follow-up. I’ve been searching high and low for any toxicology references or justifications through interpretations from government agencies for why ethylene glycol, even in a diluted form, would drop that hazard and I’ve been unable to find anything so far.
I’m beginning to think that the non-compliance I was informed of was incorrectly interpreted by my contact and I might actually be missing a precautionary or warning statement pertaining to the target organ toxicity.
That is exactly the type of references I’ve been using as well as finished vehicle coolant SDSs using ethylene glycol that have those same warnings. It is the symbol seen below that is in question right now:

This indicates the hazard class of specific organ toxicity when combined with the specific language like in your SDS snapshot where it calls out specific organs it can affect like the kidneys.
If the interpretation I’m being supplied with at this time is right, they are claiming it is unnecessary. But like I said, I can’f find any reference anywhere to back up an omission like that.
Is the DLA in your organization or a regulatory position? Just trying to understand.
DLA is a US government agency. They are in charge of reviewing, approving, purchasing, and distributing materials for the defense department. Our product was submitted by the 3rd party for review and the reviewer indicated the SDS was non-compliant and sent it back for revision.
Understood. Can you attach the page of yours in question?
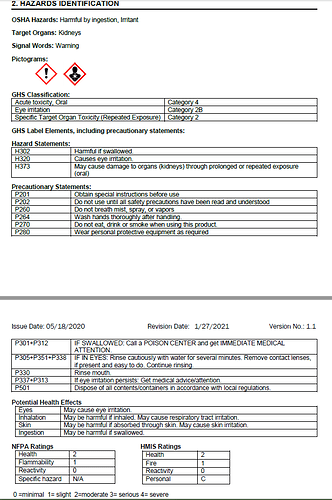
Yeah, here is the entire Section 2: Hazards Identification of the SDS which is the only segment they are saying is non-compliant:
Additionally here is Section 11: Toxicology Information which there are no notes regarding non-compliance and specifically calls out the organ toxicity int he chronic toxicity:
This is why I think the interpretation I received is incorrect and I’m being led down the wrong rabbit hole by my contact. Still no answers on that front though.
Yours is fine! I’d include a few other sources that support yours and send it back through.
1 Like
Ok, thanks for the assurance that I’m not crazy here. This is all coming at me this morning so I’ve been a bit panicked trying to get in front of it. I’ve run it by some non-technical colleagues and they all understood my stance, but they aren’t really versed in this stuff so I really wanted an informed opinion that I’m not totally bonkers for questioning this.
I’m going to ask for some clarification and if needed, references to justify the change if they insist it must be made.
I’m more hoping it is a misunderstanding and it is simply a warning phrase omission that OSHA requires or something along those lines.
Thanks for your opinion and advice.
Dunno, Super. I tend to err on the side of more information is better, but perhaps they want the general infantryman with IQ of 70 to be able to read and understand, and so a truncated version is better?
That said, I had a colleague who worked in a sister company, where hydrotesting using glycol was a regular practice…and vapor clouds from ruptured lines too. He developed some severe eye and apparent allergic reactions over the years, which were only much later attributed to glycol exposure. I had suggested replacement of the fluids used in R+D to glycerin and/or propylene glycol, both of which are far less toxic, only to be pooh-poohed. :-(
1 Like
PG can even be used in foods, if food grade. Most of our EG/water “antifreeze” systems have been converted to PG/water because it’s much safer.
I have prepared SDS’s and yours looks good to me. It certainly is not “non-compliant”. Call the guy up. He is more likely to tell you what he wants in a phone call than in an e-mail.
I do notice that you used the signal word “Warning” rather than “Danger”. That may not match with the pictograms. I would have to look that up.
1 Like
When I checked the criteria for both, the “Warning” signal word was appropriate for this one rather than “Danger”, as far as I could tell, but I would certainly not turn down a second opinion.
So I looked at it again to refresh why we went with Warning instead of Danger.
As I read it, Danger is required for target organ toxicity when the route of exposure includes transdermal, whereas respiratory and oral exposure hazards require only the Warning signal word.
I only pointed it out because the Lyondell SDS above uses the signal word danger.
I’m pleased to provide some closure for anyone still interested in this. My SDS did have an error, but the initial interpretation I was given was a mistake. It was missing a line in the GHS classifications that is supposed to go with that pictogram, see the revision below (3rd line, specifically mentioning a category 2 specific target organ toxin).
I just received notice the DLA accepted the revision. Thanks again to everyone for your input and reassurance.